Complete The Synthesis Below By Selecting Or Drawing The Reagents.
Organic Reagents
By James Ashenhurst
Reagent Friday: Raney Nickel
Last updated: January 29th, 2020 |
Raney Nickel And The Desulfurization Of Thioacetals + Hydrogenation Of Alkenes (and Alkynes)
In a blatant plug for the Reagent Guide and the Reagents App for iPhone, each Friday I profile a different reagent that is commonly encountered in Org 1/ Org 2.

Named reagents have a slightly mysterious air to them, conjuring up (for me, anyway) the image of a lone scientist working long hours for an elusive goal, until they finally have that "Eureka" moment. In this vein, while "Rearden metal" may solely be a work of fiction, "Raney Nickel" is very real. Raney nickel is an alloy of aluminum and nickel, which has subsequently had much of the aluminum removed through a leaching process with sodium hydroxide (NaOH). The remaining alloy has a very high surface area and also contains hydrogen gas (H2) adsorbed on the nickel surface.
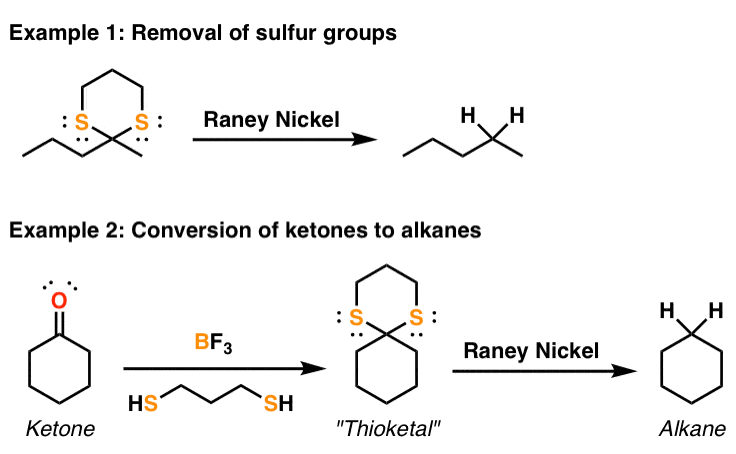
Reduction Of Sulfur Groups (Dithianes) To Alkanes With Raney Nickel
What it's used for: Like palladium on carbon (Pd/C) and platinum on carbon (Pt/C), Raney nickel can be used for the hydrogenation of alkenes and alkynes. But what Raney nickel is used most for is its unusual property of reducing C-S bonds to C-H bonds. It's this second application that can make this reagent uniquely useful. When combined with the formation of a thioketal from a ketone, this can serve as an alternative means of converting ketones to alkanes (just like the Wolff-Kishner reaction). For a real-life application of this reagent in the synthesis of erythromycin by Nobel Laureate R.B. Woodward, see the great discussion by B.R.S.M. here. Added 2019: We miss you BRSM!
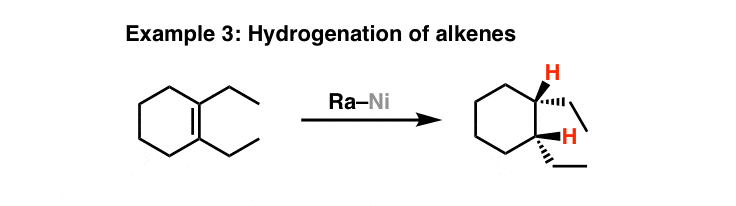
As A Hydrogenation Catalyst
Here's the second application of this reagent – as a catalyst for hydrogenation. Note that in this case we don't necessarily need to add hydrogen gas (although it helps) – Raney nickel is usually obtained in its "activated" form, where the hydrogen is already adsorbed onto it.
How it works: "For our purposes" (love this phrase) it's not so important exactly how Raney nickel works. There's something mysterious about it: the aluminum is crucial for its activity, and the metal doesn't behave the same once it's been completely removed. To be honest I plead ignorance on exactly how Raney nickel works its desulfurizing magic, although the catalytic hydrogenation process is likely similar to those of Pd/C and Pt/C.
Real life tips: Perhaps a better description for Raney nickel is "Raney Mud", because that's what it looks and feels like. Raney nickel resembles a kind of mud or wet clay, and is actually stored in water. Determining the exact molar ratio of Raney nickel to use is also something of an art – rather than "moles", typical procedures call for "teaspoons" [I'm not sure I'm aware of any other reagent that calls for this unit of measurement!] After dispensing (but before placing in the reaction vessel) the metal is then rinsed with water (to remove aluminum salts and ensure neutral pH). This can be something of a dicey prodedure since Raney nickel will spontaneously combust in air when traces of moisture are removed. Excess Raney nickel on benchtops, spatulas, weighing paper, etc. should be (carefully) destroyed with acid. There's nothing like setting up your reaction and then, out of the corner of your eye, noticing little flames coming from traces of Raney nickel on your weighing paper.
Disclaimer: this paragraph should not suffice as training in the use of this reagent, which can be extremely dangerous in the wrong hands. Don't be stupid.
P.S. You can read about the chemistry of this reagent and more than 80 other reagents in undergraduate organic chemistry in the "Organic Chemistry Reagent Guide", available here as a downloadable PDF. The Reagents App is also available for iPhone, click on the icon below!
![]()
Image credit
(Advanced) References And Further Reading
- CYCLIC KETONES FROM 1,3-DITHIANE: CYCLOBUTANONE
D. Seebach, A. K. Beck
Org. Synth. 1971, 51, 76
DOI: 10.15227/orgsyn.051.0076The method of reducing a carbonyl group to a methylene via a thioketal is known as a Mozingo reduction, and this can be done with Raney-Ni. This has applications in organic synthesis, as shown in the following references: - A One-Pot Formal [4 + 2] Cycloaddition Approach to Substituted Piperidines, Indolizidines, and Quinolizidines. Total Synthesis of Indolizidine (−)-209I
Shanghai Yu, Wei Zhu, and, and Dawei Ma
The Journal of Organic Chemistry 2005 70 (18), 7364-7370
DOI: 1021/jo051080y
The last step in the total synthesis of the title compound involves reduction of the 1,3-dithiolane with Raney Ni. - C−H Acetoxylation‐Based Chemical Synthesis of 17 β‐Hydroxymethyl‐17 α‐methyl‐18‐norandrost‐13‐ene Steroids
Alaksiej L. Hurski, Maryia V. Barysevich, Tatsiana S. Dalidovich, Marharyta V. Iskryk, Nastassia U. Kolasava, Dr. Vladimir N. Zhabinskii, Prof. Dr. Vladimir A. Khripach
Chem. Eur. J. 2016, 22 (40), 14171-14174
DOI: 10.1002/chem.201602957
Steps 30 -> 32 in this synthesis involve a Mozingo reduction. - Desulfurization of Thioketals into Methylene and Methyl Derivatives: Nickel or not Nickel?
Guangkuan Zhao, Ling‐Zhi Yuan, Mouad Alami, Olivier Provot
Chemistry Select 2017, 2 (33), 10951-10959
DOI: 1002/slct.201702370
This modern review outlines the wide variety of methods that are now available for reduction of thioacetals.This thioacetal methodology was first discovered by Nobel Laureate Prof. E. J. Corey (Harvard) and Prof. Dieter Seebach (ETH Zurich) while Prof. Seebach was a postdoc in Prof. Corey's group. Profs. Corey and Seebach coined the term 'umpolung' to describe the 'reversal of polarity' that is enabled by this method. Normally acyl groups are thought of as electrophilic (e.g. acyl cation equivalents in Friedel-Crafts acylation), but this allows one to develop syntheses based around acyl anion equivalents. - Carbanions of 1,3‐Dithianes. Reagents for C-C Bond Formation by Nucleophilic Displacement and Carbonyl Addition
E.J. Corey, D. Seebach
Angew. Chem. Int. Ed. 1965, 4 (12), 1075-1077
DOI: 10.1002/anie.196510752 - Synthesis of 1,n‐Dicarbonyl Derivates Using Carbanions from 1,3‐Dithianes
J. Corey, D. Seebach
Angew. Chem. Int. Ed. 1965 4 (12), 1077-1078
DOI: 10.1021/anie.196510771
Complete The Synthesis Below By Selecting Or Drawing The Reagents.
Source: https://www.masterorganicchemistry.com/2011/09/30/reagent-friday-raney-nickel/
Posted by: grissomfrinslazince.blogspot.com

0 Response to "Complete The Synthesis Below By Selecting Or Drawing The Reagents."
Post a Comment